half life formula for zero order reaction
For a general reaction. Given below is the half-life of a zero.
Ppt Summary Of The Kinetics Of Zero Order First Order And Second Order Reactions Powerpoint Presentation Id 545041
Remember the half-life of a reaction changes with the order of the reaction.
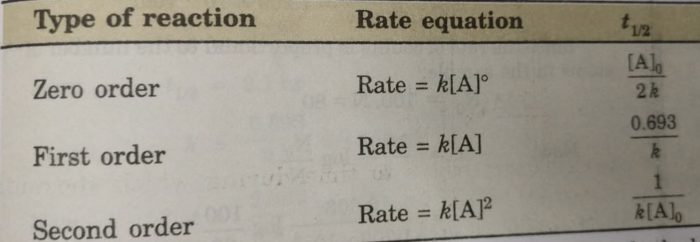
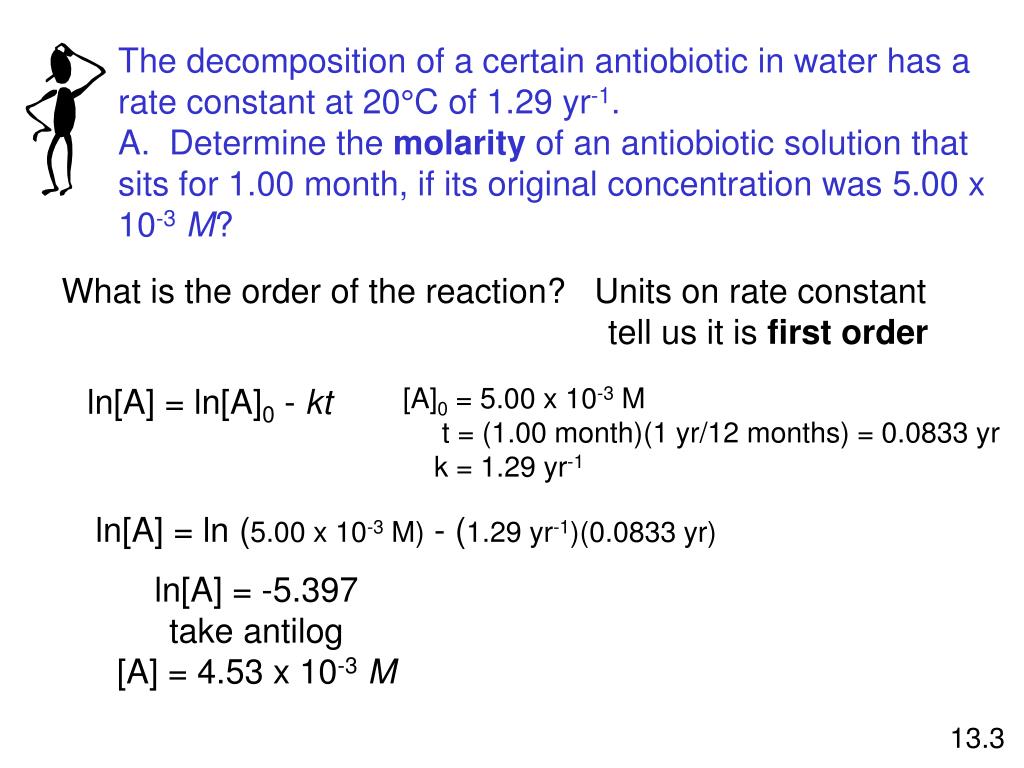
. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k For the first-order reaction the half-life is defined as t12 0693k. Half-life formula and unit for zero order reaction. A much faster rate as the graph shows.
The rate constant for a Zero-order reaction rate of constant k. A_t ktA_0 For a half-life tt_frac12. The unit of half-life equation for zero order.
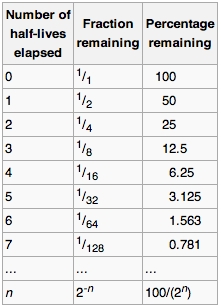
Hence the half-life of this particular radioactive substance is 3465 years. Percent completion 0053 M 52 10 5. The percent completion after 10 half-lives will be as follows.
The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction is given as t12 0693k The half-life of a second-order reaction is given by the. It is to be noted that the half-life of a zero-order reaction is determined by the initial concentration and rate constant. Zero-Order Reactions As for other reaction orders an equation for zero-order half-life may be derived from the integrated rate law.
Here k is the rate constant A is the preexponential factor Ea is the energy of activation R is the ideal gas constant and T is. For a zero order reaction k frac left Rright_ 0-left Rright t k tR0 R At t t_ 12quadleft Rrightfrac 1 2left Rright_ circ t12 R 21 R. I Arrhenius equation is kAe EaRT or lnklnA RTE a.
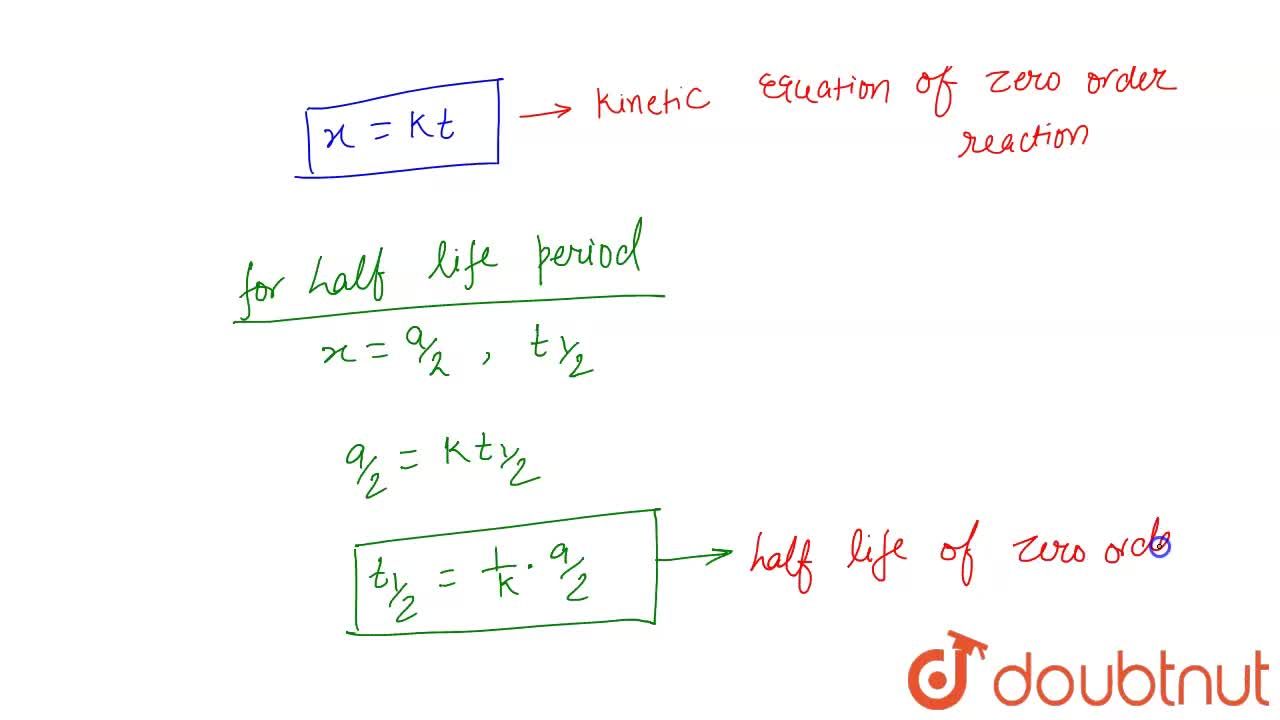
Consequently the half life equation becomes. The half-life of the reaction is denoted by t 12 and is expressed in seconds. A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant.
The half-life formula used to calculate zero order reaction is t₁₂ A₀2k. Half Life of Zero Order Reaction calculator uses Half Life of Zero Order Reaction Initial Concentration for Zero Order Reaction 2 Rate Constant of Zero Order Reaction to calculate. Percent completion 0053 M 00017 M 100 0053 97.
Half life formula for nth order reaction. We can also note that the length of half-life increase while the concentration of substrate constantly decreases unlike zero and first order reaction. Tfrac12 0693 λ tfrac12 06930002 3465.
In the case of a zero-order reaction Half-life decreases with decreasing concentration In the case of a first-order reaction Half-life is constant First-order reactions are represented by. The half-life of a zero-order reaction the formula is given as t 12 R 02 k The half-life of a first-order reaction is given as t 12 0693k The half-life of a second-order reaction.
Zero Order Half Life Pharmacokinetics
Half Life Introduction To Chemistry Course Hero
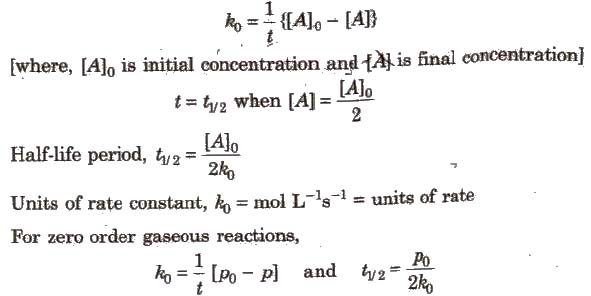
Cbse Notes Class 12 Chemistry Chemical Kinetics

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube

Welcome To Chem Zipper Com Kinetics For Nth Order Reaction

First Order Reaction Overview Equation What Is Rate Law Equation Video Lesson Transcript Study Com

Solved Half Life Equation For First Order Reactions T 2 Chegg Com
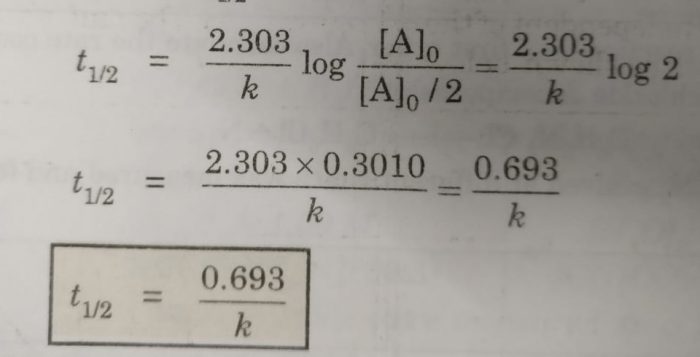
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12
What Is Half Life Period Calculate The Half Life Period For Zero Order Reaction

Half Life Of A Zero Order Reaction Is 250sec T75 T100 Of The Reaction Respectively In Sec Are Edurev Neet Question
Half Life Of Zero Order Reaction Definition Derivation Graphical Representation And Numericals Chemistry Notes
2 10 Zero Order Reactions Chemistry Libretexts
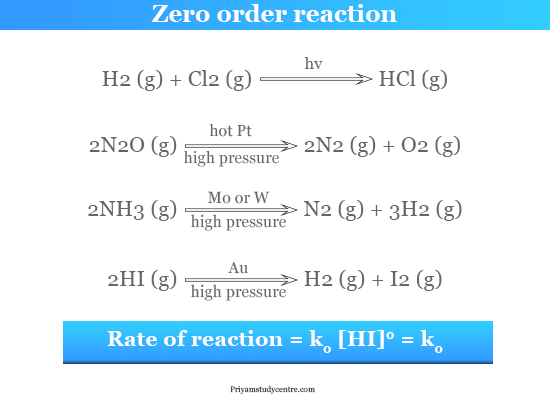
Zero Order Reaction Definition Examples Formula

How To Calculate Half Life For Zero Order Reactions Youtube
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition


